The CFR Title 21 Part 820 Memory Jogger – GOAL/QPC
Por um escritor misterioso
Last updated 10 novembro 2024

Check out the Table of Contents and selected pages at the link below.
The CFR Title 21 Part 820 Memory Jogger is an exact copy of the US Government Code of Federal Regulations U.S. Food & Drug Administration Title 21 Food and Drugs, Chapter I - Food and Drug Administration Department of Health and Human Services, Subchapter H Medical Devices, Part 820, Quality System Regulation, Medical Devices, Current Good Manufacturing Practice, April 1, 2021 Edition.
The pocket size (3.5 x 5.5) is easy to carry around and the spiral binding makes for easy reading as it will stay flat and open on a desk etc. for reviewing. The book is available for individual purchase or in a four pack or six pack at a 33% discount.
GOAL/QPC is also offering the purchaser of this book one year of free access to the digital version of this CFR Title 21 Part 820 Memory Jogger. With just a few clicks on your phone, tablet, or computer you can read through chapters or jump right to the section you are looking for with ease. You can also easily resize the text to suit your viewing style, make notes, and add links to examples, either private or across a group. Never be without your CFR Title 21 Part 820 Memory Jogger again!
GOAL/QPC can also customize this CFR Title 21 Part 820 Memory Jogger for you for free with a minimum purchase order of 25 copies. Our design team can add your logo to the cover, redesign the covers to suit your company branding, add messages from your CEO to the inside cover, add your Quality Policy to back cover, etc.
ISBN Pocket size (3.5 x 5.5): 978-1-57681-245-7
ISBN Pocket size (3.5 x 5.5 4 Pack): 978-1-57681-251-8
ISBN Pocket size (3.5 x 5.5 6 Pack): 978-1-57681-249-5
Note: GOAL/QPC makes no claim to the original US FDA works that may be contained within this book. While the contents of this book are up to date with the April 1st, 2021, edition from the U.S Government, the CFRs are continually revised and as such they may differ from what is contained in this printed book.

Ultimate Guide to 21 CFR Part 820 — FDA's Quality System Regulation (QSR) for Medical Devices

FDA 21 CFR Part 820: 8 Most Common Mistakes to Avoid

CSSBB 2002 001 PDF, PDF, Six Sigma
CSC411_Winter_2018/Project_3_Bonus/new_fake.txt at master · joshxinjie/CSC411_Winter_2018 · GitHub

Compliance with 21 CFR Part 11 – Strategies and Practices - Agaram Tech
The office theme song cello sheet music
Goal/QPC: : Books

The CFR Title 21 Part 820 Memory Jogger (4 pack)
Title 21 CFR Part 11 Compliance - testRigor AI-Based Automated Testing Tool

Ultimate Guide to 21 CFR Part 820 — FDA's Quality System Regulation (QSR) for Medical Devices
Your 1 Minute Guide to the Protection of Records – 21 CFR Part 11.10(c) [Video] - LearnGxP: Accredited Online Life Science Training Courses
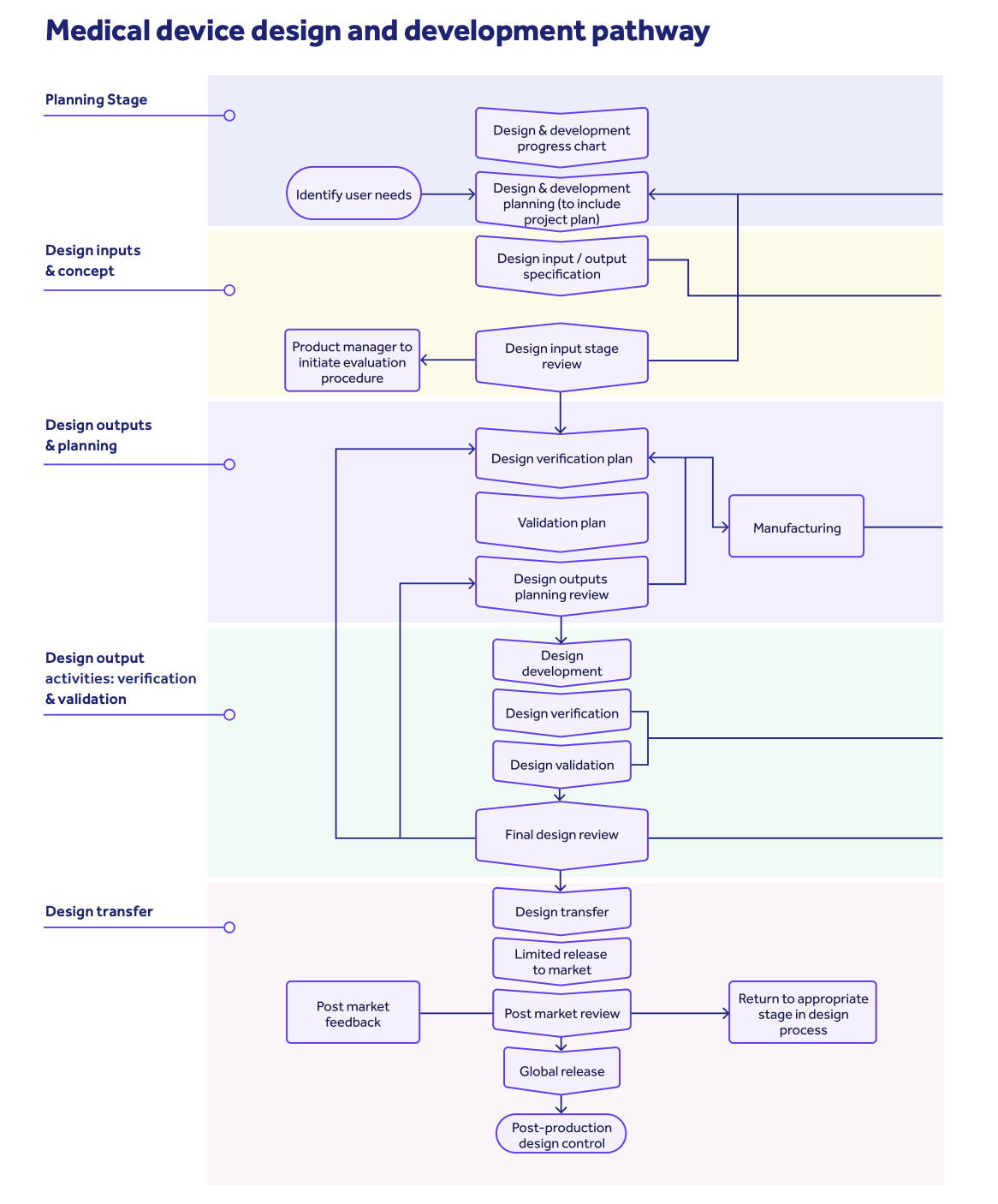
21 CFR Part 820: the complete overview

An overview of 21 CFR Part 820

The CFR Title 21 Part 11, 210 & 211, 820 Memory by Goal/QPC
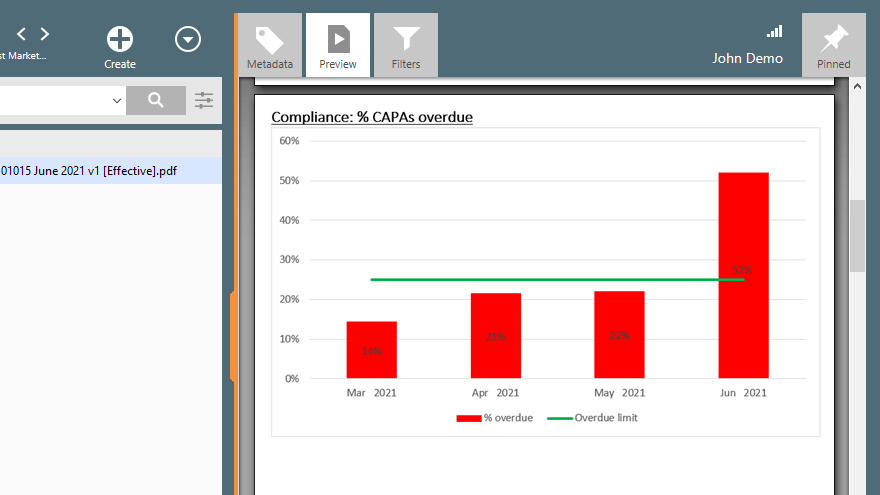
21 CFR Part 820 Quality System Regulation [Role of an eQMS]
Recomendado para você
-
Logo Memory : Food Edition - Apps on Google Play10 novembro 2024
-
 Logo Memory Challenge: Food Edition10 novembro 2024
Logo Memory Challenge: Food Edition10 novembro 2024 -
 MEM Food, Memory & Cognitive Support, 1.12 lb (510 g), California Gold Nutrition : Health & Household10 novembro 2024
MEM Food, Memory & Cognitive Support, 1.12 lb (510 g), California Gold Nutrition : Health & Household10 novembro 2024 -
 Memory Challenge Cooking Edition10 novembro 2024
Memory Challenge Cooking Edition10 novembro 2024 -
The Food Memory Project10 novembro 2024
-
 Brain Fuel: Supercharge Your Brain, Improve Memory and Lose Weight Eating Genius Foods, Expanded 2nd Edition eBook : Systems, Brain Fuel: Kindle Store10 novembro 2024
Brain Fuel: Supercharge Your Brain, Improve Memory and Lose Weight Eating Genius Foods, Expanded 2nd Edition eBook : Systems, Brain Fuel: Kindle Store10 novembro 2024 -
 Strawberry Shortcake Edition Memory Game10 novembro 2024
Strawberry Shortcake Edition Memory Game10 novembro 2024 -
 MEM Food, Memory & Cognitive Support, 60 Packets, 0.3 oz (8.5 g) Each, California Gold Nutrition10 novembro 2024
MEM Food, Memory & Cognitive Support, 60 Packets, 0.3 oz (8.5 g) Each, California Gold Nutrition10 novembro 2024 -
Take a stroll down memory lane with our Compact Electronic Grill Hot Plate Pancake Maker Takoyaki Maker Pokemon edition! 🌈✨ This…10 novembro 2024
-
 Khabaar: An Immigrant Journey of Food, Memory, and Family (Audible Audio Edition): Madhushree Ghosh, Deepti Gupta, Blackstone Publishing: Books10 novembro 2024
Khabaar: An Immigrant Journey of Food, Memory, and Family (Audible Audio Edition): Madhushree Ghosh, Deepti Gupta, Blackstone Publishing: Books10 novembro 2024
você pode gostar
-
 Herobrine - Minecraft Guide - IGN10 novembro 2024
Herobrine - Minecraft Guide - IGN10 novembro 2024 -
 Crystal onix Pokemon GO Amino10 novembro 2024
Crystal onix Pokemon GO Amino10 novembro 2024 -
 Mallyk Nagib on X: 🔴⚪🐉 ATENÇÃO! Juiz Paulo Sérgio da Silva10 novembro 2024
Mallyk Nagib on X: 🔴⚪🐉 ATENÇÃO! Juiz Paulo Sérgio da Silva10 novembro 2024 -
Milly Winky // COMMISSIONS OPEN (@TheWinkyFox) / X10 novembro 2024
-
 2 Gamepads com Gatilho Controle Celular Joystick Suporte Jogo10 novembro 2024
2 Gamepads com Gatilho Controle Celular Joystick Suporte Jogo10 novembro 2024 -
 Dominó Tátil - Brinquedos Pé de Jacaré10 novembro 2024
Dominó Tátil - Brinquedos Pé de Jacaré10 novembro 2024 -
 CULT OF THE LAMB10 novembro 2024
CULT OF THE LAMB10 novembro 2024 -
 Saitama vs Garou Manga Panel Chapter 162 One punch man manga, One punch man, One punch man anime10 novembro 2024
Saitama vs Garou Manga Panel Chapter 162 One punch man manga, One punch man, One punch man anime10 novembro 2024 -
 Sk8 Infinity Design Book, Sk8 Infinity Manga Book10 novembro 2024
Sk8 Infinity Design Book, Sk8 Infinity Manga Book10 novembro 2024 -
 Koninklijke HFC - Ex-Internationals KNVB 2024 Dates and Itineraries10 novembro 2024
Koninklijke HFC - Ex-Internationals KNVB 2024 Dates and Itineraries10 novembro 2024


