ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Last updated 10 novembro 2024


Effect of body mass index on treatment response of biologic/targeted-synthetic DMARDs in patients with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis. A systematic review - ScienceDirect

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis

Assessment of SpondyloArthritis international Society criteria for 20%
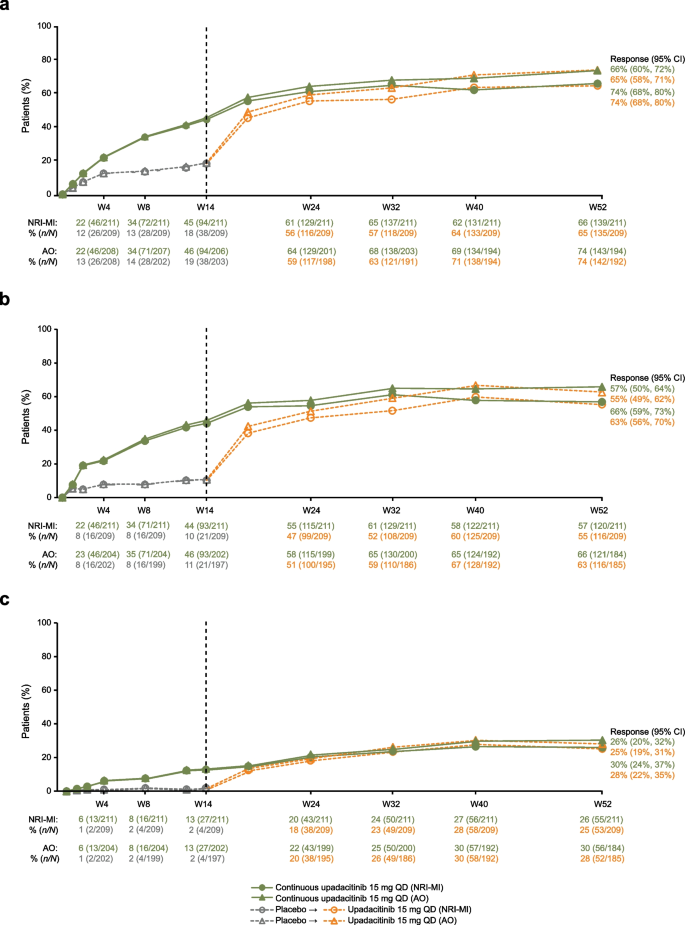
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy
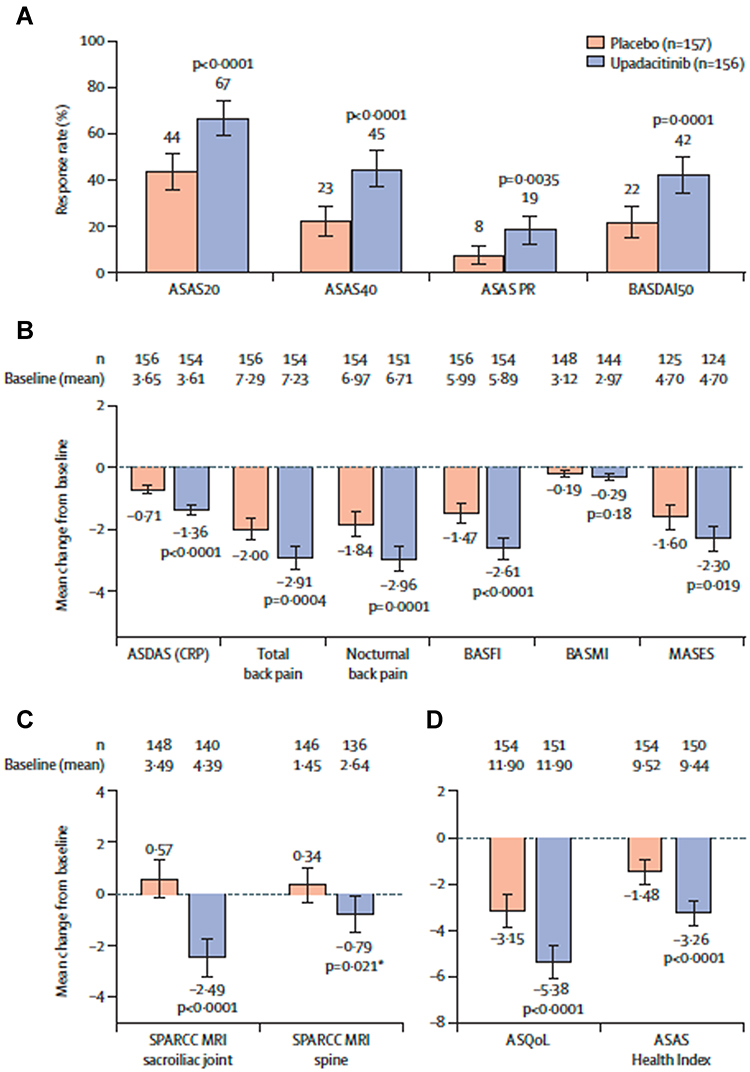
Management of axial spondyloarthritis
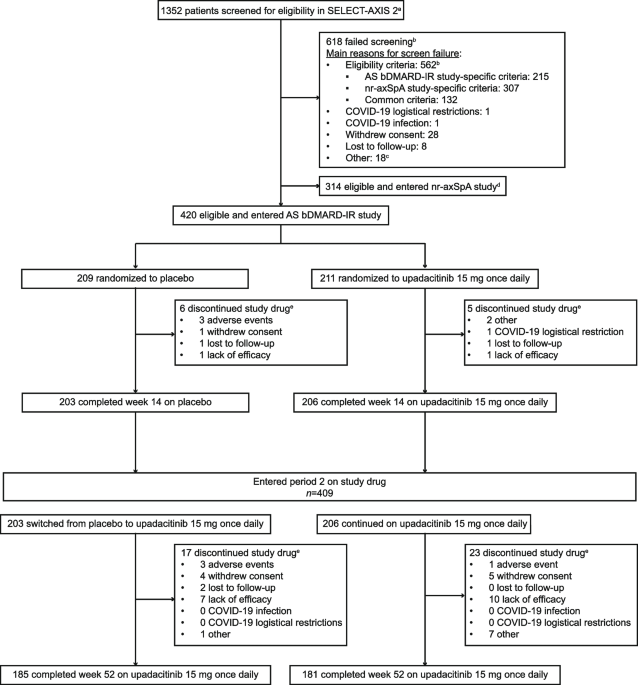
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy

Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial
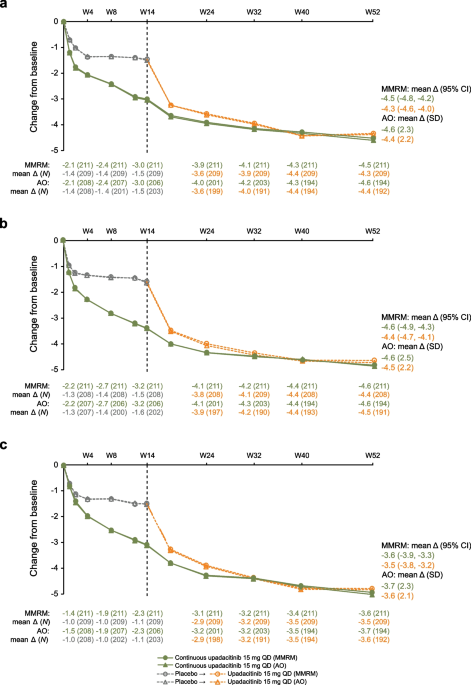
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy

PDF) Treatment response and drug retention rates in 24 195 biologic-naïve patients with axial spondyloarthritis initiating TNFi treatment: routine care data from 12 registries in the EuroSpA collaboration
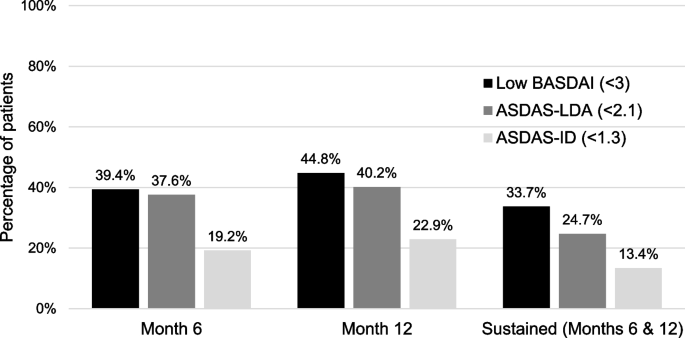
Sustained low functional impairment in axial spondyloarthritis (axSpA): which are the primary outcomes that should be targeted to achieve this?, Arthritis Research & Therapy

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis
Recomendado para você
-
ASAS_APP Did you know that ASAS has an app to facilitate the calculation of ASDAS? – click on this link to find out - ASAS - Assessment of SpondyloArthritis international Society10 novembro 2024
-
 ASAS App - ASAS10 novembro 2024
ASAS App - ASAS10 novembro 2024 -
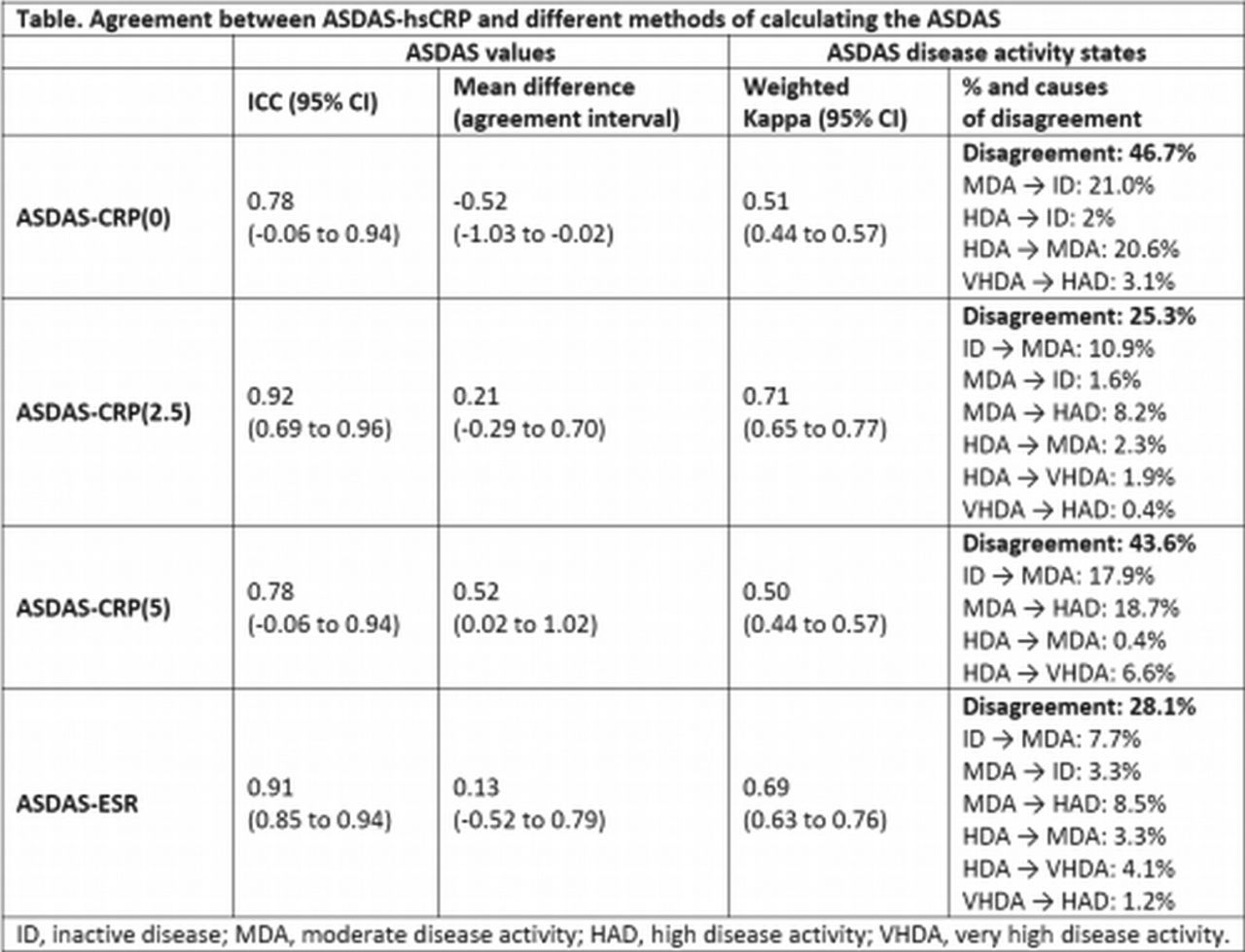 FRI0126 The Ankylosing Spondylitis Disease Activity Score (ASDAS): Defining the Best Calculation Method When the Conventional C-Reactive Protein (CRP) is below the Threshold of Detection - Results from the DESIR Cohort10 novembro 2024
FRI0126 The Ankylosing Spondylitis Disease Activity Score (ASDAS): Defining the Best Calculation Method When the Conventional C-Reactive Protein (CRP) is below the Threshold of Detection - Results from the DESIR Cohort10 novembro 2024 -
![Scoring of disease activity using BASDAI and ASDAS method in ankylosing spondylitis].](https://d3i71xaburhd42.cloudfront.net/78fc15e3e8d403a79b972c5153e6c117273eaaed/4-Table4-1.png) Scoring of disease activity using BASDAI and ASDAS method in ankylosing spondylitis].10 novembro 2024
Scoring of disease activity using BASDAI and ASDAS method in ankylosing spondylitis].10 novembro 2024 -
Bland–Altman plot for ASDAS-Q and ASDAS-CRP. ASDAS, Ankylosing10 novembro 2024
-
A. Mean ASDAS and B. mean BASDAI to week 96. Safety set (N = 89).10 novembro 2024
-
 Asda reduces emissions by 16% over 12-month period - edie10 novembro 2024
Asda reduces emissions by 16% over 12-month period - edie10 novembro 2024 -
 Asdas10 novembro 2024
Asdas10 novembro 2024 -
 Asda reveals real reason they use self-checkout cameras and it's not about data - Mirror Online10 novembro 2024
Asda reveals real reason they use self-checkout cameras and it's not about data - Mirror Online10 novembro 2024 -
 ASDAS Change status for completers over seven years. Change status of10 novembro 2024
ASDAS Change status for completers over seven years. Change status of10 novembro 2024
você pode gostar
-
 The Wild At Heart nominated in two categories for the SXSW Gaming Awards10 novembro 2024
The Wild At Heart nominated in two categories for the SXSW Gaming Awards10 novembro 2024 -
 Oliver Reed Editorial Stock Photo - Stock Image10 novembro 2024
Oliver Reed Editorial Stock Photo - Stock Image10 novembro 2024 -
VAPORIZED Robert Tercek Keynote Speech at Istanbul Innovation Week10 novembro 2024
-
 Happy Birthday to Kazuma's VA Jun! : r/Konosuba10 novembro 2024
Happy Birthday to Kazuma's VA Jun! : r/Konosuba10 novembro 2024 -
Turismo Astúrias. Áreas de Autocaravanas nas Astúrias. - Turismo10 novembro 2024
-
 Necrozma (Dusk Mane) - Moveset & Best Build for Ranked Battle10 novembro 2024
Necrozma (Dusk Mane) - Moveset & Best Build for Ranked Battle10 novembro 2024 -
 Run Away If You Can Angels of Death10 novembro 2024
Run Away If You Can Angels of Death10 novembro 2024 -
 Ender Minecraft Skins10 novembro 2024
Ender Minecraft Skins10 novembro 2024 -
![Forza Motorsport 6 Gameplay (Xbox One X HD) [1080p60FPS]](https://i.ytimg.com/vi/PMJCXQcJViU/sddefault.jpg) Forza Motorsport 6 Gameplay (Xbox One X HD) [1080p60FPS]10 novembro 2024
Forza Motorsport 6 Gameplay (Xbox One X HD) [1080p60FPS]10 novembro 2024 -
 FINAL FANTASY XIV Online - Free Trial10 novembro 2024
FINAL FANTASY XIV Online - Free Trial10 novembro 2024
